This webpage is intended for UK healthcare professionals. If you are not a healthcare professional, please click here.
Introducing Metoject® PEN
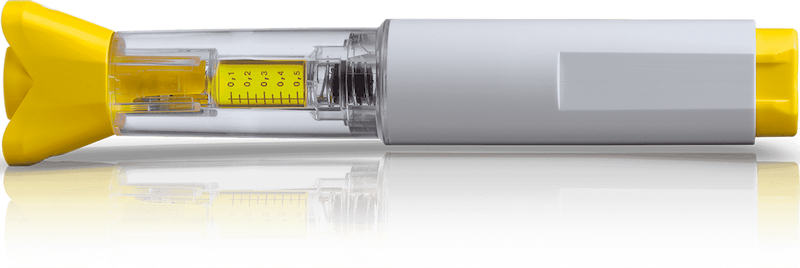
Metoject® PEN is a once a week pre-filled methotrexate auto-injector, indicated for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, psoriasis and Crohn’s disease1.
Evidence supports subcutaneous methotrexate as more efficacious and tolerable than orally administered methotrexate2, making Metoject® PEN a suitable first choice for eligible patients.
Key Benefits of Metoject® PEN Compared to Oral Administration
Greater Bioavailability
Subcutaneous administration of methotrexate offers more reliable, dose-proportional exposure which does not plateau3.
Improved GI Tolerability in Patients
Adverse effects related to the frequency and intensity of gastrointestinal discomfort were lesser in patients treated with subcutaneous methotrexate4.
Treatment Categories
Please select the relevant link below for Metoject® PEN usage information for dermatology or rheumatology.
Support Materials
Metoject® PEN Support Materials & Information
Reporting Side Effects
Adverse events should be reported using the forms and information found at yellowcard.mhra.gov.uk
Please also report any adverse events by email to medac drug safety at drugsafety@medac.de
Further Information & Questions
References:
- Metoject® Summary of Product Characteristics. [online] Available at: https://www.medicines.org.uk/emc/search?q=metoject#gref/. Last Accessed May 2023
- Müller, R.B. et al. (2015). Effectiveness, tolerability, and safety of subcutaneous methotrexate in early rheumatoid arthritis: A retrospective analysis of real-world data from the St. Gallen cohort. Seminars in Arthritis and Rheumatism, 45(1), pp.28–34.
- Pichlmeier, U., & Heuer, K. U. (2014). Subcutaneous administration of methotrexate with a prefilled autoinjector pen results in a higher relative bioavailability compared with oral administration of methotrexate. Clinical and experimental rheumatology, 32(4), 563–571.
- Kromann, C.B. et al. (2014). Does switching from oral to subcutaneous administration of methotrexate influence on patient reported gastro-intestinal adverse effects? Journal of Dermatological Treatment, 26(2), pp.188–190.
- EU Council Directive 2010/32/EU on the prevention of sharps injuries in the hospital and healthcare sector
- Latex data on file
- Braun, J. et al. (2008). Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, phase IV trial. Arthritis and rheumatism, 58(1), 73–81.
- Methotrexate and its use in rheumatoid arthritis (RA). [online] Available at: https://nras.org.uk/resource/methotrexate/. Last Accessed May 2023.
- Johnson, T.M. et al. (2021). Investigating changes in disease activity as a mediator of cardiovascular risk reduction with methotrexate use in rheumatoid arthritis. Annals of the Rheumatic Diseases, [online] 80(11), pp.1385–1392.
- O’Connor, A. et al. (2016). The rapid kinetics of optimal treatment with subcutaneous methotrexate in early inflammatory arthritis: an observational study. BMC Musculoskeletal Disorders, 17(1).
- Kanwar, A., Yadav, S. and Dogra, S. (2010). Psoriasis: What is new in nonbiologic systemic therapy in the era of biologics? Indian Journal of Dermatology, Venereology, and Leprology, 76(6), p.622.
- Warren, R.B. et al. (2017). An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet, 389(10068), pp.528–537.
- Reich, K. et al. (2020). The value of subcutaneous vs. oral methotrexate: real‐world data from the German psoriasis registry PsoBest. British Journal of Dermatology, 184(4), pp.765–767.
- Generali, E. et al. (2021). Non-adherence and discontinuation rate for oral and parenteral methotrexate: A retrospective-cohort study in 8,952 patients with psoriatic arthritis. Journal of Translational Autoimmunity, 4, p.100113.